Recombinant hCG

Recombinant Human Chorionic Gonadotropin (hCG) from Scripps Laboratories is offered as a high-quality replacement for native hCG. Starting materials for native proteins continue to be in short supply, so Scripps Laboratories is producing renewable, recombinant alternatives to address this global concern. The data presented here for Recombinant hCG demonstrate it possesses the physical characteristics to make it a suitable replacement for Native hCG.
RECOMBINANT hCG DATA
View data as a PDF: Recombinant hCG data
View products: Recombinant hCG, ≥95% | Recombinant hCG, ≥80% | Recombinant hCG, Crude Grade
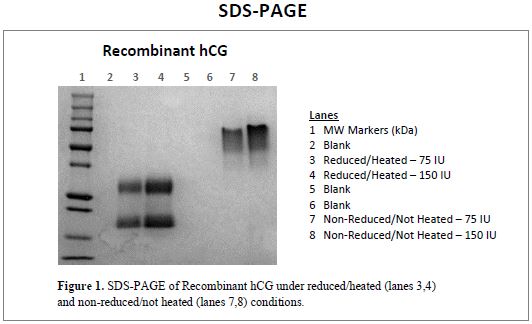
SDS-PAGE
Figure 1 presents SDS-PAGE images of Recombinant hCG, Mat. No. C0733-90646. Analysis of the gels reveals the expected molecular weight profile for hCG, showing the Recombinant α-hCG and ß-hCG subunits, as well as the intact Recombinant hCG molecule, under reduced/heated and non-reduced/not heated conditions.
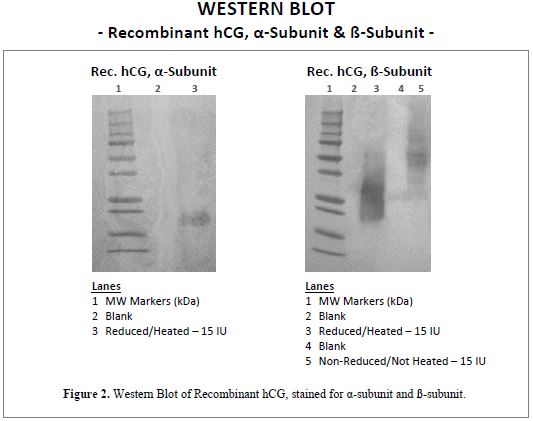
WESTERN BLOT
Recombinant hCG is the heterodimer form of hCG, composed of an α-subunit and a ß-subunit. This is confirmed in the Western Blot data presented in Figure 2. The gel on the left is stained with a monoclonal antibody specific for α-hCG and that on the right is stained with a monoclonal specific for ß-hCG. Together, the blots show the presence and antibody-reactivity of the α- and ß-subunits of Recombinant hCG.
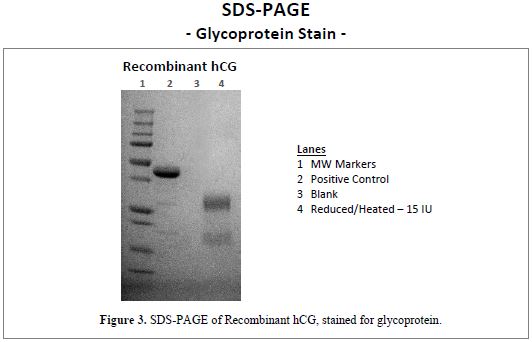
SDS-PAGE, GLYCOPROTEIN STAIN
Native hCG is a glycoprotein produced and glycated in the trophoblastic cells of the placenta during pregnancy. Recombinant hCG from Scripps Laboratories is also a glycoprotein, as can be seen at right. Figure 3 is an SDS-PAGE of Recombinant hCG, stained for glycoprotein. The gel shows clearly that the recombinant α- and ß-subunits are glycated, as expected.
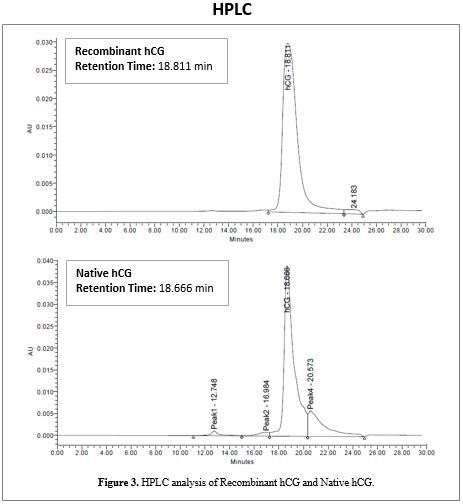
HPLC
Analyzed by HPLC, the profiles for Recombinant hCG and Native hCG show very similar retention times, indicating the two molecules have similar molecular weights.
Figure 4 shows that Recombinant hCG eluted at 18.811 minutes, while Native hCG eluted at 18.666 minutes. The difference between these retention times is <0.8%, which is well within assay-to-assay and lot-to-lot variation. This indicates the molecular weights of Recombinant hCG and Native hCG are virtually identical.
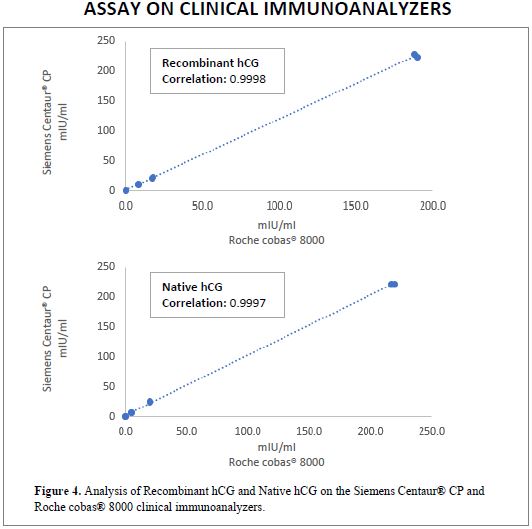
CLINICAL IMMUNOANALYZER
Recombinant hCG and Native hCG from Scripps Laboratories were analyzed on two different clinical immunoanalyzers. Analysis was performed on a Siemens Centaur® CP and a Roche cobas® 8000. Bio-Rad Lyphochek® controls were used on both instruments.
As shown in Figure 4, the correlation of both hCG products on the two clinical analyzers is excellent. Recombinant hCG showed a correlation of 0.9998, while that for Native hCG was 0.9997. Assays were run in duplicate.
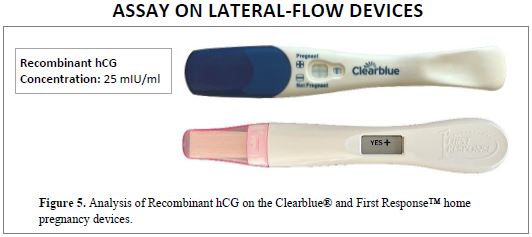
LATERAL-FLOW DEVICES
Recombinant hCG from Scripps Laboratories is also reactive in lateral-flow assay systems, as demonstrated with the Clearblue® and First Response™ home pregnancy devices. In Figure 5, positive results were obtained at a concentration of 25 mIU/ml.
The data presented here indicate Recombinant hCG is comparable to Native hCG in a variety of evaluation systems and that it is a sensible alternative to the native protein.
The molecular weight of the recombinant whole molecule, plus that for the α- and ß-subunits, display as expected. Furthermore, Recombinant hCG exhibits a retention time upon HPLC that is nearly identical to that for Native hCG.
In antibody-based assay systems, Recombinant hCG stains as expected for the α-hCG and ß-hCG subunits by Western Blot and it displays excellent reactivity on multiple clinical immunoanalyzers and lateral-flow devices.
Recombinant hCG is in stock now and available in three grades:
C0733-90646 - Recombinant hCG, ≥95%
C0732-90645 - Recombinant hCG, ≥80%
C0732-90645 - Recombinant hCG, Crude Grade
