Scripps News Volume 16 Number 2
Improving the Clinical Utility of the Serum PSA Test
The addition of assay information from biological factors -- such as prostate volume and serum levels of a PSA precursor protein -- significantly increases the sensitivity and specificity of serum PSA determinations.
In 1986, the FDA cleared the use of serum PSA testing to monitor the progression of prostate cancer in men already diagnosed with the disease. Not long after, in 1994, the FDA cleared the PSA test for use in conjunction with the digital rectal exam (DRE) to screen asymptomatic men for prostate cancer.(1) What followed was an influx of PSA tests in the clinical diagnostics market, a dramatic surge in PSA testing, and an ensuing increase in the number of prostate cancer diagnoses. (See graph below.) Along with the increased number of prostate cancer cases being detected came a wealth of new clinical data to be examined and re-examined in an effort to assess the clinical utility of the PSA test.
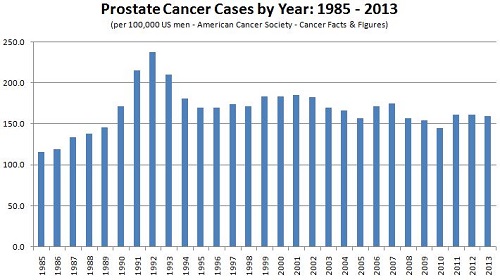
The surge in prostate cancer diagnoses was attributable to PSA tests detecting small tumors, most of which were confined to the prostate (local disease). Most of these tumors were asymptomatic, but they resulted in elevated serum PSA levels which prompted tissue biopsies to confirm the presence of cancer. Biopsy of the prostate is not a simple procedure, however, and is associated with a number of complications. Most notable of these are urinary incontinence, problems with bowel function, erectile dysfunction, and infection.(1,2)
Merely 25% of prostate biopsies are positive for cancer so the false-positive rate of the test is rather high. As such, a large number of unnecessary biopsies are performed each year, and the quality of life of a very large number of men is adversely affected.(3,4) The American Cancer Society estimates that approximately 233,000 new cases of prostate cancer will be diagnosed in the U.S. in 2014. This represents only 25% of the total number of biopsies performed, meaning nearly 700,000 men are likely to suffer unnecessarily the complications associated with prostate biopsy. This unfortunate situation has not gone unnoticed, as the U.S. Preventive Services Task Force recently recommended against the use of the PSA test as screening tool for the general male population.(2) (Of note, the recommendation does not apply to the use of the assay for patient follow-up after prostate cancer diagnosis or treatment.)
Of the biomarkers used at present to diagnose and/or monitor various types of cancer, PSA is largely considered to be the best. It does, however, have its shortcomings. Its high false-positive rate is due in part to several benign conditions that can elevate an individual’s serum PSA level. The most common such afflictions are benign prostatic hyperplasia (BPH), prostatitis (inflammation due to prostate infection), and urinary tract infection. Serum PSA levels are also affected by various drugs, such as finasteride (Proscar/Propecia) and dutasteride (Avodart), which can cloud diagnostic and prognostic evaluations.(5)
Given the challenges with interpreting serum PSA levels, researchers have been looking for ways to enhance the PSA test in an effort to better stratify men who are at risk for developing prostate cancer. Individuals with PSA developing cancer, while those with PSA >10.0 ng/ml are considered at high risk. Men who fall in the intermediate range of PSA 4.1-10 ng/ml, however, are considered to be in a “gray zone” and are more difficult to classify.(6,7,8) To improve the diagnostic and prognostic proficiency of the PSA test, for men in the gray zone in particular, a number of biological parameters are under investigation. Those receiving much of the attention are PSA velocity, percentage of free PSA vs. complexed PSA, PSA precursor proteins (pro-PSA), and PSA density.
PSA Velocity
PSA velocity (PSAV) is the rate of change of serum PSA over time, after at least three serial measurements. In general, increasing PSAV is thought to be indicative of prostate cancer rather than benign disease.(9,10) PSA levels fluctuate, however, due to the benign conditions referred to earlier, making it difficult to establish threshold values for PSAV that definitively distinguish prostate cancer from benign disease.(11,12,13) As such, PSAV as a screening tool is useful but limited.
Percentage of Free PSA to Complexed PSA
The percentage of free PSA to complexed PSA (Free/Total PSA) is determined by comparing the amount of serum PSA that is bound to a protease inhibitor, primarily α1-antichymotrypsin, with the amount of unbound, free PSA. Although not an absolute, it is generally accepted that individuals with prostate cancer produce a higher percentage of complexed PSA and a lower percentage of free PSA than do individuals with BPH, prostatitis, or urinary tract infections.(14,15) Due to this difference, the ratio of Free/Total PSA has been used for several years to increase the sensitivity of prostate cancer detection when serum PSA is in the normal range of total PSA is in the gray zone of 4.1-10 ng/ml.(12,13,16,17)
In a landmark study, Catalona et al. found that men with PSA levels in the gray zone and with Free/Total PSA <10% had a 56% likelihood of having cancer. In contrast, men with PSA levels in the gray zone and Free/Total PSA >25% had only an 8% probability of having cancer.15 As is the case with PSA, however, no single threshold value for Free/Total PSA can distinguish prostate cancer from benign disease with complete accuracy.(12,13) As such, the search continues for a better means of risk stratification.
pro-PSA
pro-PSA is a term used to describe the collection of inactive precursor proteins of PSA. Mature PSA is an enzymatically active 237-amino acid protein, but it is synthesized as a longer molecule, called pre-pro-PSA, which is cleaved to the inactive pro-PSA immediately after translation. There are several isoforms of pro-PSA, each designated by the number of amino acids it contains beyond the 237 of mature PSA.(18,19,20) The pro-PSA isoforms range from containing 2 to 7 additional amino acids, but it is the isoform with 2 extra amino acids, called [-2]pro-PSA, that has received much attention of late as it has been shown to correlate closely with the presence of prostate cancer.(21,22)
[-2]pro-PSA is part of the Prostate Health Index (phi), which is an assay system that takes serum measurements of [-2]pro-PSA and Free/Total PSA, then mathematically calculates the probability of the presence of cancer. The phi test received Premarket Approval from the FDA in June, 2012 for the testing of men age 50 and older with serum PSA values in the gray zone and a normal DRE. Several recent studies have shown that phi outperformed serum PSA and Free/Total PSA tests in differentiating prostate cancer from benign disease.(20,21,23,24) In addition, the ratio of [-2]pro-PSA to Free PSA has been shown to identify aggressive cancers and to detect cancer in men with PSA 2.5-10 ng/ml and normal DRE findings.(25,26)
PSA Density
PSA density (PSAD) is a measure of serum PSA divided by the prostate volume, as determined by transurethral ultrasound (TRUS). As early as 1992 research suggested PSAD could aid in the differentiation of prostate cancer from benign disease: PSAD values >0.15 ng/ml/ml were found to correlate with individuals with cancer, while lower values were indicative of BPH.(27,28) Subsequent studies, however, failed to show that PSAD could clearly delineate these two patient populations and, as a result, interest in PSAD slowed considerably.(29,30)
These early studies tended to assess the value of PSAD as a replacement for serum PSA in screening for prostate cancer, rather than using it to help stratify patients with elevated PSA who are candidates for biopsy or radical prostatectomy. Of late, PSAD has been evaluated in conjunction with parameters such as Gleason score, extracapsular disease, seminal vesicle invasion, lymph node invasion, and pathologic tumor upgrading.
 A number of recent studies have measured the ability of PSAD to predict an upgrade in the clinical stage of prostate cancer, as determined by Gleason scores taken after biopsy and again after radical prostatectomy. The Gleason grading system is the widely accepted standard for evaluating the stage of prostate cancer. (See inset at right.)
A number of recent studies have measured the ability of PSAD to predict an upgrade in the clinical stage of prostate cancer, as determined by Gleason scores taken after biopsy and again after radical prostatectomy. The Gleason grading system is the widely accepted standard for evaluating the stage of prostate cancer. (See inset at right.)
In short, a numerical score in the range of 2-10 is assigned to a given biopsy specimen, with higher numbers indicating a greater likelihood of advanced disease. It is a reliable predictor of disease progression and is used to direct surgical technique (e.g., whether or not to spare lymph nodes or nerve bundles).(31,32,33) In addition, an upgrade in Gleason score (GS) from initial biopsy to surgical excision of the prostate is a significant predictor of aggressive disease.(34) Due to sampling errors, however, GS is often under-estimated at the time of biopsy, leading to mismanagement of patients with high-risk disease.
Patients with GS ≤6 are an important group clinically because they have the potential to be treated with non-invasive measures. It is also, however, the group in which tumors are frequently under-classified, so a test that can help stratify patients as either high risk or low risk would be valuable.
Oh et al. studied 505 patients with GS 6 prostate cancer who underwent radical prostatectomy. Although PSA and PSAD both correlated with tumor upgrading, PSAD was the more accurate predictor and by a statistically significant margin. The authors concluded PSAD should replace PSA in the risk stratification of GS 6 patients.(35)
Corcoran et al. studied 1516 patients, categorizing them by their initial biopsy GS and their final pathology GS: Group A had a GS of 3+3=6, later upgraded to ≥7; Group B had a GS of 3+4=7, upgraded to 4+3=7; Group C had a GS of 4+3=7, upgraded to 4+4=8.(36) PSAD was the strongest predictor of GS upgrading in Groups A and B, but not in the more advanced cancers of Group C. The authors suggested the reason for this is that higher grade tumors produce less PSA per volume, reducing the effectiveness of PSAD in later stage disease.
Sfoungaristos et al. studied several preoperative clinical and pathological factors in 302 men with localized disease who had undergone radical prostatectomy.(31) Looking for a means to predict tumor upgrading or downgrading after radical prostatectomy, the investigation evaluated preoperative PSA, prostate volume, preoperative PSAD, GS, the presence of prostatitis, and a number of other factors. The authors found prostate volume to correlate significantly with tumor upgrade in patients with a biopsy GS of ≤6. Similarly, PSAD associated very strongly with tumor upgrading in patients with GS of ≤6, but not with GS ≥7. This is consistent with the other findings presented here, as more advanced cancers likely produce less PSA per volume of prostate. The other factors studied, such as preoperative PSA, benign prostatic conditions, etc., did not correlate with tumor upgrading or downgrading.
PSAD was also shown to be a useful marker when compared to PSAV in predicting tumor upgrading in men on “active surveillance.” Active surveillance is a treatment classification for men with prostate cancer that has been identified as low-risk and unlikely to metastasize. Such men are monitored by serial DRE and PSA determinations, in addition to possible repeat biopsies. In a study involving 102 patients, Kotb et al. found PSAD to be significantly better than PSAV at predicting tumor upgrading.(37)
Chen et al. evaluated PSAD, Total PSA, Free/Total PSA, and PSAV in a study of 212 men with PSA of 4-10 ng/ml who had undergone two biopsies and had normal DRE exams.(38) Of all the markers studied, PSAD was the single best predictor of prostate cancer: a cut-off of 0.18 ng/ml/ml yielded 77% sensitivity and 69% specificity.
In a novel 1999 study, PSAD was determined by measuring the volume of the prostate transition zone instead of the entire prostate as in the earlier work. The transition zone (TZ) is the inner portion of the prostate that surrounds the urethra, and evidence suggests TRUS measurement of the TZ is more accurate than that of the whole prostate.(39)
Two recent studies evaluated PSA transition zone density (PSATZD) in patients who had undergone TRUS and prostate biopsy, categorizing them by their serum PSA levels. Tang et al. divided their study population into two groups: those with PSA in the gray zone of 4.0-10.0 ng/ml and those with 10.1-20.0 ng/ml.(40) A PSATZD threshold of 0.37 ng/ml/ml provided 68.8% sensitivity and 72.6% specificity in diagnosing prostate cancer in the gray zone group, while a cut-off of 0.50 ng/ml/ml gave 70.8% sensitivity and 70.1% specificity in the 10.1-20.0 ng/ml group. The authors concluded that PSATZD measurement can improve the diagnostic efficiency of serum PSA measurements and reduce the number of unnecessary biopsies.
Janane et al. used a narrower selection criterion for their study, recruiting men with PSA levels of 2.0-4.0 ng/ml. In addition, they further stratified their study population into groups with PSA values of 2.0-3.0 ng/ml and 3.1-4.0 ng/ml.(41) Using a threshold of 0.23 ng/ml/ml for the 2.0-3.0 ng/ml PSA group and 0.28 ng/ml/ml for the 3.1-4.0 ng/ml group, PSATZD demonstrated significantly better efficiency in identifying the presence prostate cancer than did serum PSA or PSAD. Again the authors concluded that PSATZD is a viable means of reducing unnecessary biopsies.
Lastly, a 2012 study of 285 patients found PSAD to be the most predictive indicator of the key pathologic characteristics associated with prostate cancer: positive surgical margins, extracapsular disease, seminal vesicle invasion, and lymph node invasion after radical prostatectomy.(42) The other diagnostic parameters measured were preoperative PSA and GS. The authors concluded that PSAD is a valuable tool to help determine risk of recurrence and to guide the follow-up of prostate cancer patients after radical prostatectomy.
Looking Forward
Despite the recent recommendations against the use of PSA as a screening tool, a wealth of data demonstrates much improved detection of prostate cancer and risk stratification when serum PSA levels are combined with other factors, such as prostate volume (PSAD and/or PSATZD), GS, and [-2]pro-PSA. The combination of these factors appears to be especially useful in the difficult to diagnose population of men with serum PSA in the gray zone of 4-10 ng/ml and GS ≤6. Future study should focus on establishing threshold values that will aid in reducing the number of cancer-negative biopsies and improve clinical outcomes.
References
1) National Cancer Institute at the National Institutes of Health: PSA Test Fact Sheet.
2) Moyer V. “Screening for prostate cancer: U.S. Preventive Services Task Force Recommendation Statement,” Ann Int Med, 2012, 157(2): 120-135.
3) Barry M. “Prostate specific antigen testing for early diagnosis of prostate cancer,” New Engl J Med, 2001, (344(18): 1373-1377
4) Thompson I, Goodman P, Tangen C, et al. “The influence of finasteride on the development of prostate cancer,” N Engl J Med, 2003, (366): 1047-8
5) Thompson I, Pauler D, Goodman P, et al. “Prevalence of prostate cancer among men with a prostate specific antigen level ≤4.0 ng/ml,” N Engl J Med, 2004, 350(22): 2239-2246.
6) Polascik T, Oesterling J, Partin A. “Prostate specific antigen: a decade of discovery - what we have learned and where we are going,” J Urol, 1999, (162): 293.
7) Carroll P, Coley C, McLeod D, et al. “Prostate specific antigen best practice policy-part I: early detection and diagnosis of prostate cancer,” Urology, 2001, (57): 217.
8) Carter H. “Prostate cancers in men with low PSA levels - must we find them?” N Engl J Med, 2004, (350): 2292.
9) Smith D and Catalona W. “Rate of change in serum prostate specific antigen levels as a method for prostate cancer detection,” J Urol, 1994, (152): 1163.
10) D’Amico A, chen M, Roehl K, et al. “Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy,” N Engl J Med, 2004, (351): 125-35.
11) Riehmann M, Rhodes, P, Cook T, et al. “Analysis of variation in prostate specific antigen values,” Urology, 1993, (42): 390.
12) Hoffman R, Clanon D, Littenberg B, et al. “Using the free-to-total prostate specific antigen ratio to detect prostate cancer in men with nonspecific elevations of prostate specific antigen levels,” J Gen Intern Med, 2000, (15): 739.
13) Lee R, Localio A, Armstrong K, et al. “A meta-analysis of the performance characteristics of the free prostate specific antigen test,” Urology, 2006, (49): 379.
14) Lilja H, Christensson A, Dahlen U, et al. “Prostate specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin,” Clin Chem, 1991, (37): 1618-25.
15) Catalona W, Partin A, Slawin K, et al. “Use of the percentage of free prostate specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: a prospective multicenter trial,” JAMA, 1998, (279): 1542-7.
16) Adhyam M and Gupta A. “A review on the clinical utility of PSA in prostate cancer,” Indian J Surg Oncol, 2012, 3(2): 120-9.
17) Carter H, Partin A, Luderer A, et al. “Percentage of free prostate specific antigen in sera predicts aggressiveness of prostate cancer a decade before diagnosis,” Urology, 1997, (49): 379.
18) Makarov D, Isharwal S, Sokoll L, et al. “Pro-prostate specific antigen measurements in serum and tissue are associated with treatment necessity among men enrolled in expectant management for prostate cancer,” Clin Cancer Res, 2009, (15): 7316-21.
19) Mikolajczyk S, Marker K, Millar L, et al. “A truncated precursor form of prostate specific antigen is a more specific serum marker of prostate cancer,” Cancer Res, 2001, (61): 6958-63.
20) Mikolajczyk S, Catalona W, Evans C, et al. “Proenzyme forms of prostate specific antigen in serum improve the detection of prostate cancer,” Clin Chem, 2004, (50): 1017-25.
21) Sokoll L, Wang Y, Feng Z, et al. “[-2]Proenzyme prostate specific antigen for prostate cancer detection: a National Cancer Institute Early Detection Research Network validation study,” J Urol, 2008, (180): 539-43.
22) Semjonow A, Kopke T, Eltze E, et al. “Pre-analytical in vitro stability of [-2]proPSA in blood and serum,” Clin Biochem, 2010, (43): 926-8.
23) Catalona W, Bartsch G, Rittenhouse H, et al. “Serum proprostate specific antigen improves cancer detection compared to free and complexed prostate specific antigen in men with prostate specific antigen 2 to 4 ng/ml,” J Urol, 2003, (170): 2181-5.
24) Stephan C, Kahrs A, Cammann H, et al. “A [-2]proPSA-based artificial neural network significantly improves differentiation between prostate cancer and benign prostatic diseases,” Prostate, 2009, (69): 198-207.
25) Sokoll L, Sanda M, Feng Z, et al. “A prospective, multicenter, National Cancer Institute Early Detection Research Network study of [-2]proPSA: improving prostate cancer detection and correlating with cancer aggressiveness,” Cancer Epidemiol Biomarkers Prev, 2010, (19): 1193-1200.
26) Le B, Griffin C, Loeb S, et al. “[-2]Proenzyme prostate specific antigen is more accurate than total and free prostate specific antigen in differentiating prostate cancer from benign disease in a prospective prostate cancer screening study,” J Urol, 2010, (183): 1355-9.
27) Benson M, Whang I, Olsson C, et al. “The use of prostate specific antigen density to enhance the predictive value of intermediate levels of serum prostate specific antigen,” J Urol, 1992, (147): 817.
28) Andriole G, Telle W, Coplen D, et al. “PSA index (PSAI) as a predictor of prostate cancer in men with persistent serum PSA elevation,” J Urol, 1992, (147): 387A.
29) Bare R, Hart L, McCullough D. “Correlation of prostate specific antigen and prostate-specific antigen density with outcome of prostate biopsy,” Urology, 1994, (43): 191.
30) Catalona W, Richie J, deKernion J, et al. “Comparison of prostate specific antigen concentration versus prostate specific antigen density in the early detection of prostate cancer: receiver operating characteristic curves,” J Urol, 1994, (152): 2031.
31) Sfoungaristos S and Perimenis P. “Clinical and pathological variables that predict changes in tumor grade after radical prostatectomy in patients with prostate cancer,” Can Urol Assoc J, 2013, (7): E93-7.
32) Gleason D and Mellinger G. “Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging,” J Urol, 1974, (111): 58-64.
33) Hull G, Rabbani F, Abbas F, et al. “Cancer control with radical prostatectomy alone in 1,000 consecutive patients,” J Urol, 2002, (167): 528-34.
34) Corcoran N, Hong M, Casey R, et al. “Upgrade in Gleason score between prostate biopsies and pathology following radial prostatectomy significantly impacts upon the risk of biochemical recurrence,” BJU Int, 2011, 108(8 pt 2): E202-10.
35) Oh J, Hong S, Lee B, et al. “Prostate specific antigen vs prostate specific antigen density as a predictor of upgrading in men diagnosed with Gleason 6 prostate cancer by contemporary multicore prostate biopsy,” BJU Int, 2012, 110(11 pt B): E494-9.
36) Corcoran N, Casey R, Hong M, et al. “The ability of prostate specific antigen (PSA) density to predict an upgrade in Gleason score between initial prostate biopsy and prostatectomy diminishes with increasing tumor grade due to reduced PSA secretion per unit tumor volume,” BJU Int, 2012, 110(1): 36-42.
37) Kotb A, Tanguay S Luz M, et al. “Relationship between initial PSA density with future PSA kinetics and repeat biopsies in men with prostate cancer on active surveillance,” Pros Can Pros Dis, 2011, (14): 53-7.
38) Chen C, Wang S, Li J, et al. “PSA density as a better predictor of prostate cancer than percent-free PSA in a repeat biopsy,” J Chin Med Assoc, 2011, 74(12): 552-5.
39) Zlotta A, djavan B, Damoun M, et al. “The importance of measuring the prostatic transition zone: an anatomical and radiological study,” BJU Int, 1999, 84(6): 661-6.
40) Tang P, Du W, Xie K, et al. “Transition zone PSA density improves the prostate cancer detection rate both in PSA 4.0-10.0 and 10.1-20.0 ng/ml in Chinese men,” Urol Concol, 2011, Aug 23.
41) Janane A, Hajji F, Ismail T, et al. “Usefulness and predictive value of PSA density, adjusted by transition zone volume, in men with PSA levels between 2 and 4 ng/ml,” Actas Urol Esp, 2012, 36(2): 93-8.
42) Sfoungaristos S and Perimenis P. “PSA density is superior than PSA and Gleason score for adverse pathologic features prediction in patients with clinically localized prostate cancer,” Can Urol Assoc J, 2012, (6)1: 46-50.
Author contact information:
David A. George
dgeorge@scrippslabs.com
