Scripps News Volume 16 Number 1
Prolactin & Prolactin Signaling Pathways in Cancer Risk & Tumorigenesis
Prolactin is implicated in several types of cancer, with serum elevations in some and tissue expression in nearly all.
Prolactin, or luteotropic hormone, is a single polypeptide chain that is best known to initiate and sustain lactation, maintain the corpus luteum, and stimulate growth and differentiation of breast tissue.1 A versatile and potent molecule, it is known to have more than 300 functions in the body. Prolactin is structurally similar to growth hormone and is involved in mediating communication among the endocrine, nervous, and immune systems.2,3 Furthermore, recent evidence demonstrates a tumorigenic role for prolactin in breast, prostate, endometrial, ovarian, and liver cancers.
Prolactin is produced chiefly by cells called lactotrophs in the anterior pituitary gland, under the control of dopamine, which is secreted from the hypothalamus and inhibits prolactin synthesis and release. Outside the pituitary, however, prolactin is known to be produced by other cell and tissue types, such as uterine endometrium (decidua), glandular and adipose breast tissue, T cell lymphocytes, the prostate, and the hypothalamus.2,4,5,6,7,8
Elevated levels of prolactin in circulation, called hyperprolactinemia, is relatively common and may be caused by a vast number of physiologic and pathologic conditions. (Tables 1 and 2.) In addition, many commonly prescribed medications are known to induce hyperprolactinemia.9
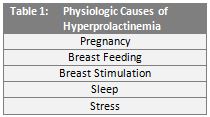
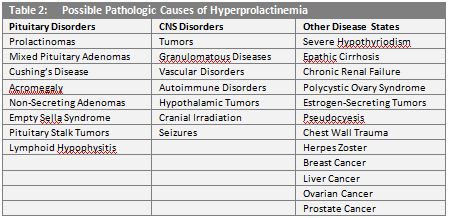
In women, hyperprolactinemia may result in irregular ovulation, menstrual abnormalities (amenorrhea or oligomenorrhea), or milk production in non-pregnant individuals (galactorrhea). In males, elevated prolactin levels are linked to impotence, diminished libido, infertility, and galactorrhea.10,11,12 While these conditions are relatively benign, prolactin has long been implicated in the spread of cancer, dating back to the 1960s and 1970s.13,14 Over approximately the last two decades, studies of prolactin-mediated signaling pathways and extra-pituitary prolactin expression have illuminated the role this powerful molecule plays in cancer progression.
Prolactin Signaling Pathways
The prolactin signaling pathways studied most often are those involving Janus Kinase-2 (Jak2) and Signal Transducer and Activator of Transcription-5 (Stat5), Ras and Mitogen Activated Protein Kinase (MAPK), and Phosphatidylinositol 3-Kinase (PI3K) and Akt kinase. These pathways are also stimulated by growth hormone and control essential cellular processes like differentiation, proliferation, survival, and motility.15,16 (Figure 1.)
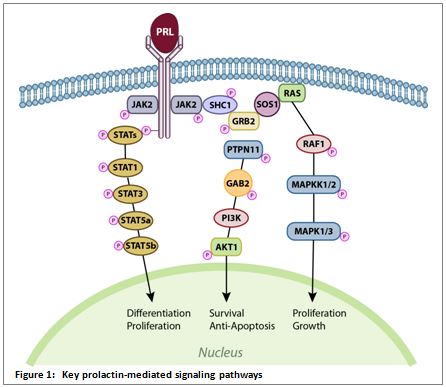
The Jak2-Stat5 cascade is one of the major signaling pathways stimulated by cytokines and growth factors. Its activation affects cell proliferation, differentiation, migration, and apoptosis.17 In breast tissue, prolactin-mediated stimulation of the Jak2-Stat5 pathway controls the majority of physiological functions in the mammary gland, such as the induction of gene transcription for proliferation/cell cycle and milk proteins.15,18,19
The Ras-MAPK signaling pathway regulates many key cellular functions, such as growth, proliferation, and apoptosis. Mutations associated with this pathway are directly linked to several types of cancer. Even in the absence of genetic aberrations, this pathway is activated in more than 50% of acute myelogenous leukemia, acute lymphocytic leukemia, and other malignancies.20,21
Activation of the PI3K-Akt pathway results in cell survival and proliferation, by modulating cell cycle regulatory proteins, and also impacts cellular migration, differentiation, and adhesion. Dysfunction of this pathway is a known causative factor in carcinogenesis. In addition, germ line mutations that affect this pathway are noted in several genetically linked forms of cancer.5,22,23
Epidemiological Studies
Prolactin’s role in tumorigenesis has been studied for quite some time in breast cancer, dating back to 1962 when Boot et al. demonstrated that high circulating levels of prolactin promoted mammary tumor growth in mouse models.13 Numerous additional studies were performed in the 1980s and 1990s, but only modest or no correlations were noted between high circulating prolactin levels and breast cancer risk.19,24 A retrospective evaluation, however, revealed that these studies were limited by a number of factors, such as small study populations, availability of post-diagnosis samples only, and only one sample being drawn from each study participant, which prevented prolactin levels from being monitored over a period of time.24,25
In a review of data obtained from the Nurses’ Health Studies (NHS) 1 and 2, which involved more than 237,000 female subjects, a link between prolactin and breast cancer has become more evident. High serum levels of prolactin were associated with unsuccessful treatment, earlier disease recurrence, and a lower overall survival rate. The review also associated elevated serum prolactin with a 60% increased risk of developing estrogen receptor-positive cancer.25
Data from other subsets of the NHS population have been analyzed, and a family history of breast cancer was associated with significantly higher prolactin levels in premenopausal women, as compared to those with no such family history.26 In another analysis, a positive correlation was seen between elevated serum prolactin levels and risk of breast cancer in both pre- and postmenopausal women. The association was slightly stronger in patients whose tumors tested positive for estrogen and progesterone receptors.27
Pathway Dysfunction in the Breast and Prostate
Supplementing these epidemiological reviews are several studies that indicate a causative role for prolactin in tumorigenesis. Prolactin mRNA and locally-produced, non-pituitary prolactin have been identified in normal and malignant breast tissue.1,4,5,28 In addition, these tissues are known to express prolactin receptors, which would suggest the possibility of mammary cells secreting prolactin molecules that would act on themselves (autocrine signaling) or neighboring cells (paracrine signaling). Indeed, autocrine and paracrine induction of prolactin-mediated signaling pathways are well-documented in breast tissue.1,4,5,28,29
Over-stimulation or other dysfunction of intracellular signaling pathways are well-known mechanisms of oncogenesis.30 Theoretically, if abnormally high levels of prolactin were present in circulation or the extracellular matrix (ECM), it could lead to aberrant stimulation of the prolactin receptor, resulting in activation of the various prolactin-mediated pathways and, ultimately, the development of cancer.
Many studies demonstrate that prolactin, whether produced locally or by the pituitary, promotes tumor growth in mammary tissue by activation of the Jak2-Stat5 and Ras-MAPK pathways.15,20,31,32,33,34,35,36,37 In a 2013 study, Barcus et al. evaluated the roles of prolactin signaling and ECM density in breast cancer invasiveness.38 The authors found that in stiff, dense collagen matrices, prolactin activated ERK1/2, a component of the Ras-MAPK pathway, and increased the expression of matrix metalloproteinase-2 (MMP-2). Both consequences of prolactin signaling increased the invasive behavior of breast cancer cells.
The PI3K-Akt pathway, although not yet directly linked to cancer by way of prolactin, does produce several downstream metabolites that regulate systems important in oncogenesis, such as proliferation, inhibition of apoptosis, angiogenesis, and cytoskeletal rearrangements.39,40,41,42 By affecting these downstream processes, prolactin may have an indirect role in tumor progression. Further study is warranted.
In addition to mammary carcinoma, prolactin mediation of the Jak2-Stat5 pathway is also implicated in the development of prostate cancer. Both prolactin and its receptor are expressed in normal and malignant prostate epithelium, suggesting the possibility of an autocrine prolactin signaling mechanism.7,43 In two separate studies, Li et al. demonstrated that Stat5 activation is associated with a higher tumor grade and earlier recurrence in prostate cancer.44,45 In a follow-up study, Dagvadorj et al. clearly demonstrated that autocrine prolactin, independent from pituitary prolactin, promotes prostate cancer cell growth by activation of the Jak2-Stat5 pathway.46
Prolactin in Other Malignancies
Evidence is mounting for a role of prolactin in at least two gynecologic malignancies: Endometrial and ovarian cancer. An exhaustive 2007 study evaluated the ability of 64 biomarkers to distinguish endometrial cancer from breast and ovarian cancers. Among all the markers, prolactin was best able to identify patients with endometrial cancer, and by quite a large margin: The prolactin assay demonstrated 98.3% sensitivity and 98.0% specificity, while the next most accurate marker exhibited 40.5% sensitivity and 95.0% specificity.47
In ovarian cancer, serum levels of prolactin do not strongly correlate with risk of disease development in the general population, but elevated levels are associated with increased risk in two population subsets: Overweight women and those with a strong family history of ovarian cancer.48,49 Furthermore, factors associated with a reduced risk of ovarian cancer, such as having borne children and the use of oral contraceptives, correlate strongly with lower prolactin levels, suggesting a role for prolactin in mediating these effects.48 Additional study is needed.
Experimental evidence suggests a causative role for prolactin in endometrial and ovarian tumorigenesis, although not as much data exists as does for breast and prostate cancers. Levina et al. found the presence of prolactin mRNA and over-expression of the prolactin receptor in ovarian and endometrial tumors.49 The authors also demonstrated prolactin’s ability to induce proliferation in endometrial and ovarian cancer cell lines. In addition, they found that prolactin was able to transform normal ovarian epithelial cells into cancerous tissue by way of Ras activation, a factor implicated in endometrial and ovarian carcinogenesis.50,51,52 More study is needed, but the involvement of prolactin in these malignancies appears likely.
Prolactin may also be involved in liver cancer, as a recent study reported significantly elevated serum prolactin levels in patients with hepatocellular carcinoma, compared to healthy controls.53 The study also evaluated prolactin receptor expression and Jak2 activation in patient tissue samples, and both were found to correlate with a poor outcome. Further analysis confirmed that prolactin induced the activation of Jak2 and cyclin D1 in hepatocellular carcinoma cells, suggesting the proliferation of liver cancer may occur by way of the Jak2-Stat5 signaling pathway.
Summary
The versatility and potency of the prolactin molecule is unquestionable. As early as 1962 it was implicated as a causative factor in mammary carcinoma. Since then, prolactin has been shown to predict treatment failure, disease recurrence, and a lower overall survival rate in breast cancer. In addition, its expression in tissue indicates tumorigenic potential and increased risk of developing disease in breast, prostate, endometrial, ovarian, and liver cancers. With prolactin’s seemingly ubiquitous synthesis and secretion, along with its involvement in multiple intracellular signaling pathways, it’s likely that even more roles and functions will be revealed in the near future.
References
1) Clevenger CV and Plank TL. “Prolactin as an autocrine/paracrine factor in breast tissue,” J Mammar Gland Biol Neoplasia, 1997, 2(1): 59-68.
2) Freeman ME, Kanyicska B, Lerant A, et al. “Prolactin: structure, function, and regulation of secretion,” Phsyiol Rev, 2000, 80(4): 1523-1537.
3) Ben-Jonathan N, LaPensee CR, and LaPensee EW. “What can we learn from rodents about prolactin in humans?” Endocr Rev, 2008, 29: 1-31.
4) Zinger M, McFarland M, and Ben-Jonathan N. “Prolactin expression and secretion by human breast glandular and adipose tissue explants,” J Clin Endocr Metab, 2003, 88(2): 689-696.
5) Chen CC, Stairs DB, Boxer RB, et al. “Autocrine prolactin induced by the Pten-Akt pathway is required for lactation initiation and provides a direct link between the Akt and Stat5 pathways,” Genes Dev, 2012, 26(19): 2154-2168.
6) Ben-Jonathan N, Mershon JL, Allen DL, et al. “Extrapituitary prolactin: distribution, regulation, functions, and clinical aspects,” Endocr Rev, 1996, 17: 639-669.
7) Clevenger CV, Furth PA, Hankinson SE, et al. “The role of prolactin in mammary carcinoma,” Endocr Rev, 2003, 24(1): 1-27.
8) Jabbour HN, Buggay O, and Chritchley H. “Prolactin action and signaling in the human endometrium,” Reprod Med Rev, 2002, 10: 117-132.
9) La Torre D and Falorni A. “Pharmacological causes of hyperprolactinemia,” Ther Clin Risk Manag, 2007, Oct, 3(5): 929-951.
10) American Society for Reproductive Medicine, “Hyperprolactinemia (Prolactin Excess),” 2007.
11) Gomez F, Reyes FI, and Faiman C. “Nonpuerperal galactorrhea and hyperprolactinemia. Clinical findings, endocrine features, and therapeutic responses in 56 cases,” Am J Med, 1977, 62(5): 648.
12) Schlecte J, Sherman B, Halmi N, et al. “Prolactin-secreting pituitary tumors in amenorrheic women: a comprehensive study,” Enocr Rev, 1980, Summer, 1(3): 295-308.
13) Boot LM Muhlbock O, and Repcke G. “Prolactin and the induction of mammary tumors in mice,” Gen Comp Endocr, 1962, 2: 601-602.
14) Welsch CW and Nagasawa H. “Prolactin and murine mammary tumorigenesis: a review,” Cancer Res, 1977, 37: 951-963.
15) Swaminathan G, Varghese B, and Fuchs SY. “Regulation of prolactin receptor levels and activity in breast cancer,” J Mammary Gland Biol Neoplasia, 2008, 13(1): 81-91.
16) Piwien-Pilipuk G, Huo JS, and Schwartz J. “Growth hormone signal transduction,” J Ped Endocr Metab, 2002, 15: 771-786.
17) Rawlings FS, Rosler KM, and Harrison DA. “The JAK/STAT signaling pathway,” J Cell Science, 2004, 117: 1281-1283.
18) Watson CJ and Burdon TG. “Prolactin signal transduction mechanisms in the mammary gland: the role of the Jak/Stat pathway,” Rev Reprod, 1996, 1: 1-5.
19) Clevenger CV, Furth PA, Hankinson SE, et al. “The role of prolactin in mammary carcinoma,” Endocr Rev, 2003, 24: 1-27.
20) Santarpia L, Lippman SL, and El-Naggar AK. “Targeting the mitogen-activated protein kinase RAS-RAF signaling pathway in cancer therapy,” Expert Opin Ther Targets, 2012, 16(1): 103-119.
21) McCubrey JA, Steelman LS, CVhappell WH, et al. “Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance,” Biochim Biophys Acta, 2007, 1773(8): 1263-1284.
22) Carnero A, Blanco-Aparicio C, Renner O, et al. “The PTEN/PI3K/AKT signaling pathway in cancer, therapeutic implications,” Curr Cancer Drug Targets, 2008, 8(3): 187-198.
23) Li Y, Podsypanina K, Liu X, et al. “Deficiency of PTEN accelerates mammary oncogenesis in MMTV-Wnt-1 transgenic mice,” BMC Mol Biol, 2001, 2:2, Epub.
24) Fernandez I, Touraine P, and Goffin V. “Prolactin and human tumorigenesis,” J Endocr, 2010, 22: 771-777.
25) Tworoger SS and Hankinson SE. “Prolactin and breast cancer etiology: an epidemiologic perspective,” J Mammary Gland Biol Neoplasia, 2008, Mar, 13(1): 41-53.
26) Eliassen AH, Tworoger SS, and Hankinson SE. “Reproductive factors and family history of breast cancer in relation to plasma prolactin levels in premenopausal and postmenopausal women,” 2007, Int J Cancer, 120(7): 1536-1541.
27) Tworoger SS, Sluss P, and Hankinson SE. “Association between plasma prolactin concentrations and risk of breast cancer among predominately premenopausal women,” Cancer Res, 2006, 66(4): 2476-2482.
28) B4 Ginsburg E and Vonderhaar BK. “Prolactin synthesis and secretion by human breast cancer cells,” Cancer Res, 1995, 55: 2591-2595.
29) Arendt LM and Schuler LA. “Transgenic models to study actions of prolactin in mammary neoplasia,” J Mammary Gland Biol Neoplasia, 2008, 13: 29-40.
30) Martin GS. “Cell signaling and cancer,” Cancer Cell, 2003, 4: 167-174.
31) Das R and Vonderhaar BK. “Activation of raf-1, MEK, and MAP kinase in prolactin responsive mammary cells,” Breast Cancer Res Treat, 1996, 40(2): 141-149.
32) Llovera M, Pichard C, Bernichtein S, et al. “Human prolactin antagonists inhibit hPRL-activated signaling pathways involved in breast cancer cell proliferation,” Oncogene, 2000, 19: 4695–4705.
33) DaSilva L, Rui H, Erwin RA, et al. “Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515 and TYR580,” Mol Cell Endocr, 1996, 117: 131–140.
34) Schaber JD, Fang H, Xu J, et al. “Prolactin activates Stat1 but does not antagonize Stat1 activation and growth inhibition by type I interferons in human breast cancer cells,” 1998, Cancer Res 58: 1914–1919.
35) Clevenger CV, Torigoe T, and Reed JC. “Prolactin induces rapid phosphorylation and activation of prolactin receptor associated Raf-1 kinase in a T-cell line,” J Biol Chem, 1994, 269: 5559–5565.
36) Das R and Vonderhaar BK, “Activation of raf-1 MEK, and MAP kinase in prolactin responsive mammary cells,” Breast Cancer Res Treat, 1996, 40: 141–149.
37) Das R and Vonderhaar BK. “Involvement of SHC, GRB2, SOS and RAS in prolactin signal transduction in mammary epithelial cells,” Oncogene, 1996, 13: 1139–1145.
38) Barcus CE, Keely PJ, Eliceiri KW, et al. “Stiff collagen matrices fuel tumorigenic prolactin signaling,” J Biol Chem, 2013, March 24, Ms M112.447631.
39) Roymans D and Slegers H. “Phosphatidylinositol 3-kinases in tumor progression,” Eur J Biochem, 2001, 268: 487–498.
40) Toker A. “Protein kinases as mediators of phosphoinositide 3-kinase signaling,” Mol Pharmacol, 2000, 57: 652–658.
41) Blume-Jensen P and Hunter T. “Oncogenic kinase signaling,” Nature, 2001, 411: 355–365.
42) Cantrell DA. “Phosphoinositide 3-kinase signaling pathways,” J Cell Sci, 2001, 114: 1439–1445.
43) Nevalainen MT, Valve EM, Ingleton PM, et al. “Expression and hormone regulation of prolactin receptors in rat dorsal and lateral prostate,” Endocr, 1996, 137: 3078-3088.
44) Li H, Ahonen TJ, Alanen K, et al. “Activation of signal transducer and activator of transcription-5 in human prostate cancer is associated with high histological grade,” Cancer Res, 2004, 64: 4774-4782.
45) Li H, Zhang Y, Glass A, et al. “Activation of signal transducer and activator of transcription-5 in prostate cancer predicts early recurrence,” Clin Cancer Res, 2005, 11: 5863-5868.
46) Dagvadorj A, Collins S, Jomain JB, et al. “Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway,” Endocr, 2007, 148(7): 3089-3101.
47) Yurkovetsky Z, Ta’asan S, Skates S, et al. “Development of multimarker panel for early detection of endometrial cancer. High diagnostic power of prolactin,” Gynecol Oncol, 2007, 107: 58-65.
48) Clenden TV, Arslan AA, Lokshin AE, et al. “Circulating prolactin levels and risk of epithelial ovarian cancer,” Cancer Causes Control, 2013, 24(4): 741-748.
49) Levina VV, Nolen B, Su YY, et al. “Biological significance of prolactin in gynecologic cancers,” Cancer Res, 2009, 69(12): 5226-5233.
50) Al-Jehani RM, Jeyarajah AR, Hagen B, et al. “Model for the molecular genetic diagnosis of endometrial cancer using K-ras mutation analysis,” J Natl Cancer Inst, 1998, 90: 540-542.
51) Esteller M, Garcia A, Martinez-Palones JM, et al. “The clinicopathological significance of K-RAS point mutation and gene amplification in endometrial cancer,” Eur J Cancer, 1997, 33: 1572-1577.
52) Pijnenborg JM, Dam-de Veen GC, Kisters N, et al. “RASSF1A methylation and K-ras and B-raf mutations and recurrent endometrial cancer,” Ann Oncol, 2007, 18: 491-497.
53) Yeh YT, Lee KT, Tsai CJ, et al. “Prolactin promotes hepatocellular carcinoma through Janus kinase 2,” World J Surg, 2012, 36(5): 1128-1135.
Author contact information:
David A. George
dgeorge@scrippslabs.com
